Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Uchida, Shunsuke*; Hanawa, Satoshi; Naito, Masanori*; Okada, Hidetoshi*; Lister, D. H.*
Corrosion Engineering, Science and Technology, 52(8), p.587 - 595, 2017/10
Times Cited Count:4 Percentile:21.41(Materials Science, Multidisciplinary)Based on the relationship among ECP, metal surface conditions, exposure time and other environmental conditions, a model to evaluate the ECP and corrosion rate of steel was developed by coupling a static electrochemical analysis and a dynamic oxide layer growth analysis. Major conclusion obtained on the model are as follows. The effect of H O
O and O
and O concentrations on ECP were successfully explained as the effects of oxide layer growth. Hysteresis of ECP under changes in water chemistry conditions were successfully explained with the model. Decreases in ECP due to neutron exposure were explained well by radiation-induced diffusion in the oxide layers.
concentrations on ECP were successfully explained as the effects of oxide layer growth. Hysteresis of ECP under changes in water chemistry conditions were successfully explained with the model. Decreases in ECP due to neutron exposure were explained well by radiation-induced diffusion in the oxide layers.
 -dimethylformamide
-dimethylformamideKim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Ikeda, Yasuhisa*
Journal of Alloys and Compounds, 408-412, p.1291 - 1295, 2006/02
Times Cited Count:10 Percentile:54.65(Chemistry, Physical)The electrochemical reactions of UO (
( -diketonato)
-diketonato) DMF, UO
DMF, UO (trop)
(trop) DMF and UO
DMF and UO (sap)(DMF)
(sap)(DMF) , (DMF = N,N-dimethylformamide,
, (DMF = N,N-dimethylformamide,  -diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E
-diketonate = thenoyltrifluoroacetonate (ttfa), benzoyltrifluoroacetonate (btfa), and dibenzoylmethanate(dbm), trop = tropolonate, and sap = 2-salicylidenaminophenolate) complexes in DMF solution containing tetrabutyl ammonium perchlorate as a supporting electrolyte have been studied with cyclic voltammetry. These uranyl(VI) complexes were found to be quasi-reversibly reduced to U(V) species. The formal redox potentials (E ,
, 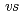 . ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO
. ferrocene/ferrocenium) for U(VI)/U(V) couples were determined to be -1.18 V for UO (ttfa)
(ttfa) DMF, -1.18 V for UO
DMF, -1.18 V for UO (btfa)
(btfa) DMF, -1.46 V for UO
DMF, -1.46 V for UO (dbm)
(dbm) DMF, -1.46 V for UO
DMF, -1.46 V for UO (trop)
(trop) DMF, and -1.59 V for UO
DMF, and -1.59 V for UO (sap)(DMF)
(sap)(DMF) .
.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji
Radiochimica Acta, 93(12), p.767 - 770, 2005/12
Times Cited Count:10 Percentile:56.74(Chemistry, Inorganic & Nuclear)Electrochemical and spectroelectrochemical has been used to investigate the behaviour of Neptunium (VI) in concentration 1-8 M HNO solutions. The electrochemical reactions of Np(VI) ions were found to occur quasi-reversibly. The formal redox potentials (E
solutions. The electrochemical reactions of Np(VI) ions were found to occur quasi-reversibly. The formal redox potentials (E ) for Np(VI)/Np(V) couples were determined to be +0.906, +0.908, +0.909, +0.902, +0.896, +0.895, +0.888, and +0.884 V (vs. Ag/AgCl) for Np(VI) ions in 1, 2, 3, 4, 5, 6, 7, 8 M HNO
) for Np(VI)/Np(V) couples were determined to be +0.906, +0.908, +0.909, +0.902, +0.896, +0.895, +0.888, and +0.884 V (vs. Ag/AgCl) for Np(VI) ions in 1, 2, 3, 4, 5, 6, 7, 8 M HNO solutions, respectively. The reduction processes of Np(VI) ions were followed spectroelectrochemically by using an optical transparent thin layer electrode cell. It was found that the absorption spectra measured at the applied potentials from +1.10 to +0.60 V versus Ag/AgCl reference electrode redox couple for Np(VI) in HNO
solutions, respectively. The reduction processes of Np(VI) ions were followed spectroelectrochemically by using an optical transparent thin layer electrode cell. It was found that the absorption spectra measured at the applied potentials from +1.10 to +0.60 V versus Ag/AgCl reference electrode redox couple for Np(VI) in HNO solution have clear isosbestic points. These results indicate that the reduction product of Np(VI) is Np(V), which is considerably stable in HNO
solution have clear isosbestic points. These results indicate that the reduction product of Np(VI) is Np(V), which is considerably stable in HNO solution.
solution.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji
JAERI-Conf 2005-007, p.341 - 344, 2005/08
Electrochemistry has been used to investigate the behavior of plutonium(IV) in 1-7 M HNO solutions. These Pu(IV) complexes were found to be reduced quasi-reversibly to Pu(III) species. The formal redox potentials (E
solutions. These Pu(IV) complexes were found to be reduced quasi-reversibly to Pu(III) species. The formal redox potentials (E ) for Pu(IV)/Pu(III) couples were determined to be +0.721, +0.712, +0.706, +0.705, +0.704, 0.694, and +0.696 V (vs. Ag/AgCl(SSE)) for Pu(IV) complexes in 1, 2, 3, 4, 5, 6, 7 M HNO
) for Pu(IV)/Pu(III) couples were determined to be +0.721, +0.712, +0.706, +0.705, +0.704, 0.694, and +0.696 V (vs. Ag/AgCl(SSE)) for Pu(IV) complexes in 1, 2, 3, 4, 5, 6, 7 M HNO solutions, respectively. These results indicate that the reduction product of Pu(IV) is Pu(III), which is considerably stable in HNO
solutions, respectively. These results indicate that the reduction product of Pu(IV) is Pu(III), which is considerably stable in HNO solution.
solution.
 and UCl
and UCl dissolved in a LiCl-KCl eutectic melt
dissolved in a LiCl-KCl eutectic meltKuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Journal of the Electrochemical Society, 152(4), p.C203 - C212, 2005/04
Times Cited Count:108 Percentile:94.63(Electrochemistry)The electrochemical behavior of UCl and UCl
and UCl dissolved in LiCl-KCl eutectic melt was studied at 723-823 K. Electroreduction of U(IV) in LiCl-KCl melt occurs via two successive steps involving transfer of one and three electrons. The diffusion coefficients of U(IV) and U(III) ions were determined by linear sweep voltammetry, chronopotentiometry, and chronoamperometry. The formal standard potential of E
dissolved in LiCl-KCl eutectic melt was studied at 723-823 K. Electroreduction of U(IV) in LiCl-KCl melt occurs via two successive steps involving transfer of one and three electrons. The diffusion coefficients of U(IV) and U(III) ions were determined by linear sweep voltammetry, chronopotentiometry, and chronoamperometry. The formal standard potential of E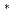 U(IV)/U(III), E
U(IV)/U(III), E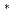 U(IV)/U, and E
U(IV)/U, and E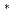 U(III)/U were determined and some thermodynamic properties of UCl
U(III)/U were determined and some thermodynamic properties of UCl and UCl
and UCl dissolved in LiCl-KCl eutectic melt were calculated. Influence of oxide ions on electrochemical behavior was also studied.
dissolved in LiCl-KCl eutectic melt were calculated. Influence of oxide ions on electrochemical behavior was also studied.
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Uchiyama, Gunzo*; Ikeda, Yasuhisa*
Radiochimica Acta, 93(2), p.75 - 81, 2005/02
Times Cited Count:9 Percentile:53.19(Chemistry, Inorganic & Nuclear)no abstracts in English
Kim, S.-Y.; Asakura, Toshihide; Morita, Yasuji; Uchiyama, Gunzo*; Ikeda, Yasuhisa*
Journal of Radioanalytical and Nuclear Chemistry, 262(2), p.311 - 315, 2004/11
Times Cited Count:11 Percentile:58.51(Chemistry, Analytical)no abstracts in English
Shirai, Osamu; Uozumi, Koichi*; Iwai, Takashi; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.745 - 748, 2002/11
no abstracts in English
Hayashi, Hirokazu; Minato, Kazuo
JAERI-Conf 2001-016, 181 Pages, 2001/12
Applications of molten salts technology to separations and syntheses of materials have been studied eagerly, which would develop new fields of materials science. Research Group for Actinides Science, Department of Materials Science, Japan Atomic Energy Research Institute (JAERI), together with Reprocessing and Recycle Technology Division, Atomic Energy Society of Japan, organized the Workshop on Molten Salts Technology and Computer Simulation at Tokai Research Establishment, JAERI on September 18, 2001. In the workshop eleven lectures were made and lively discussions were there on the bases and applications of the molten salts technology that covered the structure and basic properties of molten salts, the pyrochemical reprocessing technology and the relevant computer simulation.
Ogawa, Toru; Minato, Kazuo
Pure and Applied Chemistry, 73(5), p.799 - 806, 2001/05
Times Cited Count:15 Percentile:54.07(Chemistry, Multidisciplinary)no abstracts in English
Hayashi, Hirokazu; Minato, Kazuo*
no journal, ,
For a basis of the future nuclear fuel cycle, understanding the behavior of transuranium elements (TRU: Np, Pu, Am, Cm) is important. Experimental study on pyrochemical reprocessing of the fuels containing TRU is one of the important topics. But limited data are available on the behavior of TRU in some molten salts including NaCl-2CsCl melt which is considered to be used for pyrochemical reprocessing of MOX fuels in RIAR process. Only the electrochemical behavior of Np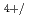 Np
Np redox couple has been reported for Np ions in NaCl-2CsCl melt. In the present study, behavior of Np ions including the reduction of Np
redox couple has been reported for Np ions in NaCl-2CsCl melt. In the present study, behavior of Np ions including the reduction of Np in NaCl-2CsCl melt was investigated. A typical cyclic voltammogram shows 2 groups of the signals corresponding to Np species. They were assigned to the redox reactions of Np
in NaCl-2CsCl melt was investigated. A typical cyclic voltammogram shows 2 groups of the signals corresponding to Np species. They were assigned to the redox reactions of Np /Np and Np
/Np and Np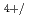 Np
Np , respectively by comparing with those observed in LiCl-KCl melt. The redox potentials and the diffusion coefficients obtained by various transient electrochemical techniques will be presented.
, respectively by comparing with those observed in LiCl-KCl melt. The redox potentials and the diffusion coefficients obtained by various transient electrochemical techniques will be presented.